Company Begins Human Trials Of Implanting Brain Chips That Let You Control Computers With Your Thoughts
Published on May 9, 2022 at 4:01 PM by Face of Malawi
238 words • approx. 2 min read
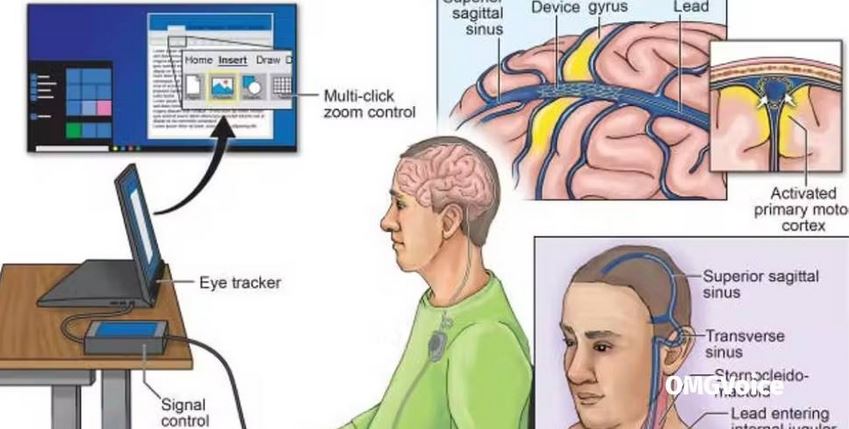
SYNCHRON has launched a clinical trial that will implant brain chips in humans in the United States.
The Stentrode brain implant – about the size of a paper clip – will let the wearer control a computer using just their thoughts.
It is reportedly being tested on six patients with severe paralysis at the Mountain Sinai Hospital in New York and at the University Pittsburgh Medical Center in Pennsylvania.
Stentrode will let patients use digital devices with their brains, allowing them to perform daily tasks such as texting and online shopping.
And while the device has already been tested on Australian subjects, this is the first time it is being tried out in the United States.
The trial, dubbed Command, is being performed under the watchful eye of the US Food and Drug Administration (FDA).
“The approval of this [clinical trial] reflects years of safety testing performed in conjunction with FDA,” Dr. Thomas Oxley, CEO of Synchron, said in a statement.
“We have worked together to pave a pathway forward, towards the first commercial approval for a permanently implanted [brain computer interface] for the treatment of paralysis.”
Oxley added that Command is a “major milestone” for the company which hopes to bring its product to the ~5 million people living with paralysis in the US.
The American Journal of Public Health has concluded that stroke is the leading cause of paralysis, followed by spinal cord injury, multiple sclerosis, and cerebral palsy.


