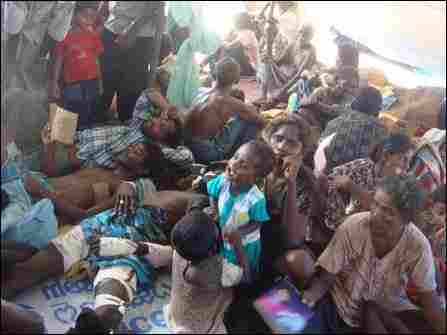
SOMETIMES, the blindingly obvious is not actually correct. Malnutrition, for instance, is obviously caused by a lack of food. And yet, as a paper published in this week’s Science by Jeffrey Gordon and his colleagues at Washington University, in St Louis, points out, that is not always a complete explanation.
Dr Gordon’s team have been looking at twins in Malawi. Two years ago they announced that despite both members of such pairs eating the same diet, one twin often remains healthy while the other becomes malnourished. This discordance is associated with differences in the gut bacteria of the individuals involved. Their latest paper explores why.
The answer seems to lie partly in the role bacteria play in providing essential nutrients to the body and partly in the inhibition of a biochemical pathway called the Krebs cycle, which is at the core of every organism’s metabolism. The Krebs cycle transfers the energy in sugar to a molecule called ATP, which is the body’s energy currency. Interfere with it and the whole metabolism will slow down, with potentially catastrophic consequences. And if a child has the wrong bacteria in his gut, that seems to be what happens.
Dr Gordon and his team followed 317 pairs of Malawian twins for the first three years of their lives. In half of these pairs, both twins thrived. In 7%, both were malnourished. In the remainder, however, one twin seemed well-fed whereas the other had symptoms of malnutrition.
Malnutrition comes in various forms. The two most severe are marasmus (a chronic, progressive wasting) and kwashiorkor (whose symptoms include oedema, and various skin and liver problems). For simplicity, the team looked at kwashiorkor.
They first established that the discordance they had observed was not related to a child’s own genetic make-up. They did this by showing that discordance is as common in fraternal twins (who share half their genes) as it is in identical twins (who share all of them). They then looked at how twins’ gut bacteria differ.
Teasing out such differences is hard. Guts harbour many bacterial species in what is known as a microbiome. But modern methods of DNA sequencing allow these species to be identified, and having done so the team were able to show that the microbiomes of children with kwashiorkor differ systematically from those of the well-nourished. What they did not know was whether these differences cause kwashiorkor or merely reflect it.
They therefore transplanted samples of faeces, microbiome and all, into mice that had been born germ free, and discovered that bacteria from children with kwashiorkor do indeed cause murine malnutrition, but only in combination with the sort of diet eaten by children in Malawi—which is basic, but not inadequate. That diet, in combination with bacteria from healthy children, produced no symptoms. Conversely, mice whose bacteria came from kwashiorkor sufferers, but which were fed what is known as ready-to-use therapeutic food (RUTF), a peanut-based concoction that is given routinely to malnourished children to fatten them up, showed no signs of malnutrition either.
Having established that bacteria can be at least part of the cause of kwashiorkor, the team studied a few cases in greater detail. They worked out which bacterial enzymes are more, or less, active in the guts of children with kwashiorkor (or, rather, in the guts of mice into which the appropriate faecal samples have been transplanted), and which metabolic products are more, or less, abundant.
Vicious cycle
Two results stood out. First, transplants from well-nourished children generated higher levels of essential amino acids in mice than those from children with kwashiorkor. (An essential amino acid is one that the human body cannot make for itself, so it must be provided either directly from food or by bacterial metabolism.) Second, an analysis of the by-products of the Krebs cycle in the mice’s urine suggested that several of the enzymes which regulate this cycle were being inhibited in the kwashiorkor-transplant mice. Both of these phenomena are likely to lead to the symptoms of malnutrition.
The team also found that the malnutrition-inducing microbiome is a resilient thing. Feeding RUTF to a mouse with such a microbiome fattened the animal up while the diet continued, but did not dislodge the microbes—so some other treatment will have to be devised. This might be a different form of therapeutic diet or it might be some way of “rebooting” the gut microbiome directly, by adding missing species or subtracting unwanted ones. This is an approach that is also being tested to treat people with a potentially lethal gut infection called Clostridium difficile.
The upshot is that malnutrition is a more complicated phenomenon than it might, at first, appear to be. This study adds to the growing science of microbiomic medicine, in which the lives of the bacterial passengers that people carry around are given due weight and consideration lest they turn on their hosts and hurt them.